Abstract
INTRODUCTION: Tyrosine kinase inhibitors (TKIs) remain mainstay in the management of patients with CML. Several TKIs have been approved over the last two decades. Even though efficacy and safety remain as the primary drivers in treatment selection, in recent years importance has been placed on treatment affordability and economic burden associated with the long-term cancer treatments such as TKIs. However, current literature lacks real-world data on healthcare utilization (HCU) and costs among patients with CML using TKIs, which this study aims to address.
METHODS: Retrospective cohort study was conducted using MarketScan Commercial, and Supplemental Medicare databases (2012-2016). Data includes medical and pharmacy utilization and costs for over 90 million individuals enrolled in employer-sponsored health-plans in the US. Data includes information on but not limited to medical diagnosis, procedures, drugs dispensed, date of service, health plan enrollment. Study involves adult patients (≥18 years) with ≥2 medical claims with a diagnosis of CML (ICD-9-CM: 205.10 - 205.12; ICD-10: C92.10-C92.12) and with a prescription claim for TKI. The date of first-TKI claim defined the index date. Patients were required to have continuous health plan enrollment ≥6 months before (defined as baseline period) and ≥6 months after index date. Selected patients were further classified into 5 sub-groups based on the index TKI observed (prescribed) post CML diagnosis (imatinib, dasatinib, nilotinib, bosutinib, ponatinib). Among the selected patients, all-cause HCU and costs were assessed from index date until end of database or end of enrollment, whichever occurred earlier. HCU and associated costs were assessed overall and by care settings including inpatient, emergency room, physician office, outpatient hospital, outpatient pharmacy, nursing facility and ancillary care. In addition, baseline (6 month) utilization and costs were assessed. Monthly and annual resource utilization and costs were reported for the overall CML cohort (across all TKI users) and by index TKI sub-groups. All costs were reported from a payer perspective (i.e., costs reimbursed by health plan) and adjusted to 2017 US dollars using the US consumer price index (medical component). All analyses were descriptive in nature.
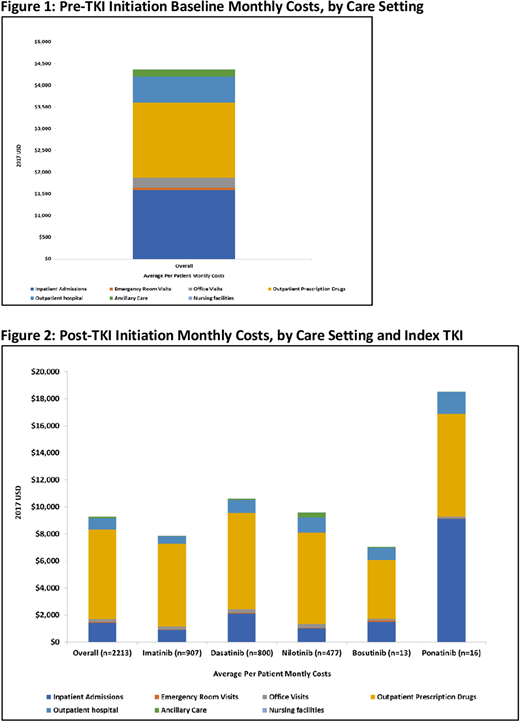
RESULTS: The study cohort included 2,213 CML patients. Distribution for the index TKI treatment was as follows: 41% imatinib, 36% dasatinib, 21% and 1% each for bosutinib and ponatinib. Mean age (standard deviation [SD]) of the cohort was 55 (15) years which was similar for individual TKI sub-groups. Majority of patients were males (55%) and 56% were enrolled in a preferred provider organization plan. The mean follow-up duration post-TKI initiation was 607 (442) days. The average baseline monthly all-cause costs were $4,365 with inpatient and pharmacy costs accounting for over 3/4th of the total costs (Figure 1). Post-TKI initiation the average monthly costs were twice ($9,288) compared with the baseline costs ($4,365) and the increase was primarily attributable to higher outpatient pharmacy costs ($6,619, accounted for 71% of total costs) (Figure 2). Monthly costs across other care settings (inpatient, outpatient, emergency room) were similar for the baseline and post-TKI initiation. On average patients had 1.4 office visits, 2.5 prescriptions and 0.7 hospital outpatient visits per month at baseline, which increased by 23%, 38% and 49%, respectively post TKI-initiation. During the 1st year post TKI-initiation, 17% patients in the overall CML cohort had at least 1 inpatient admission and this was consistent across individual TKI-sub-groups (except ponatinib, 50%).
CONCLUSIONS: Findings on TKI utilization and costs in employer-sponsored health-plan database indicate that average costs and utilization were similar across the TKI sub-groups with pharmacy costs accounting for 71% of the total post TKI initiation costs. Overall, this study helps address the literature gap by providing recent real-world treatment care-setting specific utilization and costs among TKI uses and these data can be of value to several healthcare stakeholders including physicians, managed care plans and researchers in supporting clinical and formulary decisions and also serve as inputs for economic models. Finally, the sample sizes for ponatinib and bosutinb were small and results for these TKIs should be interpreted with caution.
Huang:ZS Associates: Employment; Novo Nordisk Inc: Equity Ownership; AstraZeneca: Research Funding. Karve:AbbVie: Employment, Equity Ownership. Porwal:ZS Associates: Employment. Thakkar:ZS Associates: Employment. Marshall:AbbVie: Employment, Equity Ownership. Rosenberg:AbbVie: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal